Abstract
Background: The current standard of care in acute myeloid leukemia (AML) does not include cerebral spinal fluid (CSF) evaluation in patients without central nervous system (CNS) symptoms and current induction regimens do not include CNS prophylaxis with intrathecal chemotherapy. Although the incidence of CNS involvement in AML patients is low, further analysis is necessary to characterize and prevent this devastating complication. The aim of our study is to study specifically secondary AML patients who developed CNS metastasis and their clinical characteristics and outcomes.
Methods: This was a retrospective study of any adult AML patients who were treated and diagnosed at the multicenter Montefiore Health Care System between January 2011 to May 2022 with confirmed CNS metastasis through either imaging or pathologically from CSF testing. Overall survival (OS) defined as the time of CNS diagnosis to death in patients with CNS metastasis. When comparing those without CNS metastasis and those with CNS metastasis OS was defined as time of AML diagnosis to death date or last contact. DFS defined as the time from complete remission (CR) until the first event (relapse or death) or last contact date. Chi-square test was used to compare categorical variables and applied logistic regression model to estimate the odds ratio and 95% CI around it. Medians were compared using Mann-Whitney test. The log-rank test was used to compare survival in patients with and without CNS. Statistical analysis was conducted using R studio v4.2.1.
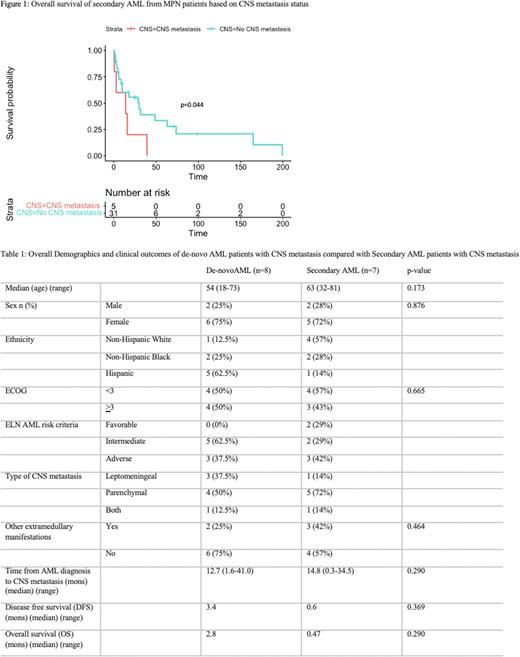
Results: In total, 1160 adult AML patients were seen between January 2011 to May 2022 with 15 (1.3%) patients found to have had CNS metastasis. The median age of these patients was 63 years old (range 18-81) along with 87% with either intermediate or adverse as classified by the ELN risk criteria and median ECOG status at start of CNS disease of 2. Of the 15 patients, 7 (47%) patients were from secondary AML patients, of which 5 (71%) patients had a precedent myeloproliferative neoplasm (MPN) diagnosis. The other 2 (29%) patients with secondary AML were from a previous diagnosis of myelodysplastic syndrome (MDS). Of the 5 patients with prior MPN, 1 had essential thrombocythemia, 1 had myelofibrosis, 1 had primary polycythemia vera and 2 had CML. The mutations in these 5 patients included 2 JAK2 V617F, 1 triple negative and 2 BCR-ABL positive. Sixty percent of patients with CNS metastasis were treated with a combination of chemotherapy and radiation therapy or chemotherapy alone. The median OS of all patients with CNS metastasis was 1.0 months and 2.8 months and 0.47 months with de-novo and secondary AML respectively but was not statistically significant (p=0.290). In secondary AML patients from antecedent MPNs, the OS was statistically significant worse in those with CNS metastasis compared to those without CNS metastasis (p=0.044) (Figure 1). Additional characteristics and outcomes summarized in Table 1.
Conclusion: Although CNS metastasis in AML is rare, this study demonstrates its impact in secondary AML patients. When specifically comparing secondary AML patients from previous MPNs diagnoses, the OS was statistically worse in those with CNS compared to those without. In comparing patients between whether they had de-novo AML or secondary AML with CNS metastasis, there was no difference in OS, possibly indicating secondary AML may not be the sole driving factor for CNS metastasis. Larger studies are needed to confirm this finding along with future prospective studies evaluating which patients are at a greater risk should receive prophylaxis or be monitored closely with periodic lumbar punctures. The next goal of this research is to determine risk factors associated with CNS involvement to identify those at high risk by multivariate analysis (akin to the CNS-IPI scoring system used in Diffuse Large B-Cell Lymphoma) in AML patients in order to properly risk stratify those who may be at a higher risk for CNS metastasis and to investigate the role of prophylactic measures to prevent these devastating and often fatal events.
Disclosures
Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal